In 1928, a farmer in Western Australia named Jack Trott was plowing a field newly carved from the Outback by fire. An unusual crack in the soil caught his attention. In it was something extraordinary — a sweet-smelling pallid little flower of the first known completely subterranean plant: the Western Underground Orchid, Rhizanthella gardneri*.
Strange things are known to lurk under Australia’s soils (these come once again to mind), and these flowers must surely be counted among their ranks (although not perhaps for much longer — fewer than 50 individuals are known to exist). Obviously, living your entire life underground is weird for a group of organisms (i.e., plants) known for its sun worship. True to their underground creds, these orchids never break the surface. Or rather, they do break it (crack it, usually), but they never break on through, in the Doors-ensian sense. The flowers form within a few inches of the surface, but get no further. The sweet smell is clearly a lure for pollinators — identity still TBD — to their underground digs.
As orchids go, they are odd in in that their “flower” is actually a cluster of flowers in a cup called a “capitulum”, or “little head”. If you look at the picture, you’ll see a bunch of upside-down teardrop shapes; those are the flowers, in which orchid afficianados can no doubt see a faint resemblance to more conventional orchids that this “flower” as a whole quite lacks.
But also weird for orchids, these flowers subvert the usual fungus-orchid BFF relationship. Many, if not most orchids, have what is usually euphemistically termed an “intimate” relationship with fungi. That starts on day one: germination. Orchids are notoriously difficult to start from seed because they store no food of their own and must be in the presence of their preferred fungal partner — under Goldilocks conditions — to sprout. For most wild orchids, your best source of information on what that fungus and those conditions might be would be your nearest Magic 8-Ball.
Most photosynthetic, self-sufficient orchids seem to associate with saprophytic or parasitic fungi. But R. gardneri has found a different niche: myco-heterotrophy, or exploiting the mycorrhizal fungi of other plants. In this case, it gets all up in the business of the fungal partners of Melaleuca uncinata, the broom bush. Mycorrhizae are fungi that encase or invade the roots of nearly every land plant. This is good; without them, plants would have a considerably rougher go of it. The usual exchange is sun-made plant glucose in exchange for a vastly improved water and mineral uptake thanks to the exponential surface area increase made possible by a network of fungal filaments extending well beyond the roots. Mycoheterotrophic plants break into the mycorrhizal associaton of a fungus and another plant and steal some of the goodies for themselves.
R. gardneri is not the first plant to have figured this sweet deal out. Here in Colorado there are at least two pale pink spiky plants terrorize our fungi: the Heath-family pinedrops, and the Orchid-family spotted coralroot. I often see pinedrops and spotted coralroot on hikes in the mountains, where the former associates with (probably parasitizes) mycorrhizal truffle fungi in the genus Rhizopogon (related to the bolete genus Suillus for those who care) and the latter goes after mycorrhizal Russula, which make the majority of the red-capped, white-stalked (and usually poisonous and/or disgusting) mushrooms found in the temperate forest. I wrote about another Heath-family floral parasite that I used to encounter often here. In spite of exceptions like pinedrops, almost all mycoheterotrophs are orchids.
In all these cases, these plants have lost their chlorophyll. But none have taken the admittedly drastic step (for a plant) of entirely forsaking wind and rain, sun and cloud.
Chloroplasts: Not Just for Photosynthesis Anymore
The Western Underground Orchid has, and in the process, has gone farther toward forsaking its planthood (if its planthood can be tied to the vir(d)ility of its chloroplasts — the cell bits responsible for photosynthesis) than any other plant.
Chloroplasts — as first understood with genius by Lynn Margulis — are what remains of a fateful meal billions of years ago. A bigger cell engulfed a smaller photosynthetic one, probably a cyanobacterium, or blue-green alga. But the alga didn’t go quietly. It managed to survive and thrive in its would-be predator. It became its partner instead. Already in that cell were the remains of bacterial cells who had long ago struck a similar bargain: mitochondria, the power-packs of the cell. You have these ancient bacteria still in nearly every cell of your body. Plants have both mitochondria (which is how we know that endosymbiotic event happened first) and chloroplasts, the descendants of the world’s luckiest alga. And both still have DNA, the remains of their bacterial chromosomes.
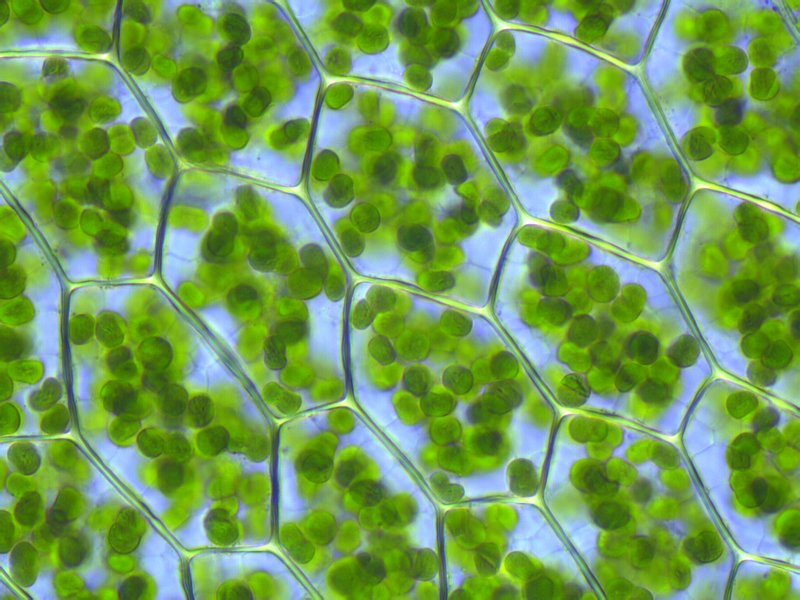
Chloroplasts inside a true moss -- each one a descendant of an alga that gave a predatory cell indigestion billions of years ago. Its offspring (and that of a few other lucky photosynthetic microbes cum organelles) blanket the planet. Creative Commons Krisitian Peters, click image for license link.
But in R. gardneri, 70% of its expected chloroplast genes are gone, according to a new paper in Molecular Biology and Evolution. And the total length of the R. gardneri’s chloroplast chromosome (which is still circular like all most good bacteria) is just 59,190 base pairs, also the smallest for a land plant on record.
Chloroplasts are where the proteins and other cogs of photosynthesis are manufactured. They’re where photosynthesis takes place too. But it seems that even chloroplasts must have some functions in the cell aside from harnessing light. Because Rhizanthella has gone as far as any plant in ditching their chloroplasts since they spend their entire lives underground, ensuring zero photosynthesis takes place. So maybe the news isn’t that they’ve shed 70 % of their chloroplasts genes. It’s that they still have 30% of them. What are those genes still doing there? And what can they tell us about how other parasites operate?
Even in your garden-variety plant, the chloroplast genome is sharply reduced compared to its cyanobacterial ancestor. When a cell goes from going it solo to living in a co-op, there’s a lot of genes it doesn’t pay to make in-house. There may be genes already doing the same thing in the host cell’s nucleus, or just not usable from inside another cell anymore. Over time, natural selection will prune these genes away, since they are costly to make if not being used. Other genes, perhaps because it’s more efficient to regulate and transcribe them there, get shuttled to and permanently housed in the nucleus. What’s left is chiefly what’s essential for the business of turning light into sugar, Job #1 for the choroplast.
But in Rhizanthella, which has clearly boarded up the photosynthetic apparatus, there remain 37 genes coding for 20 proteins, 4 ribosomal RNAs (an important constituent of ribosomes), and 9 transfer RNAs (essential tools for turning RNA into proteins within the ribosome; they ferry the appropriate amino acid to the appropriate RNA codon). Scientists checked DNA made from the messenger RNA they found in the chloroplasts; they were all being spliced and edited correctly, implying they were, in fact, still being used, in spite of the strip mining of the rest of the genome.
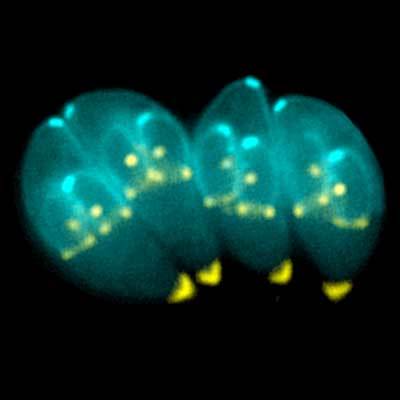
Toxoplasma gondii motherships constructing new invasion craft. Creative Commons Ke Hu and John Murray. Click image for license link.
Compare those 37 genes to a standard photosynthetic orchid, in this case Phalaenopsis aphrodite, whose chloroplast genome has 110 genes. Even the parasite Toxoplasma gondii, notorious for its mind-control abilities (which chiefly involve making hosts — including accidental hosts like humans — do risky things to increase their odds of predation, or from the parasite’s point of view, getting to the next host), actually seems to have been photosynthetic in a previous life, and even it still contains 53 genes on its presumed remnant chloroplast genome, according to the authors.
Thus ex-photosynthetic plants of all sorts seem to share a minimum set of chloroplast genes, and they must be doing so because the chloroplast performs some sort of important non-photosynthetic function that can’t be transferred to the nucleus.
In fact, the scientists compared the reduced gene sets found in parasite ex-chloroplasts from a variety of groups. As mentioned above, they found strong similarities, suggesting that when photosynthetic parasites of any ancestry — related or not — give up free-living, they tend to lose and retain the same sorts of chloroplast genes. This knowledge could provide us with lines of attack in ex-photosythetic parasites that go after us or our crops or livestock. The bare-bones Rhizanthella genes could tell us which genes are most essential — and thus the best targets.
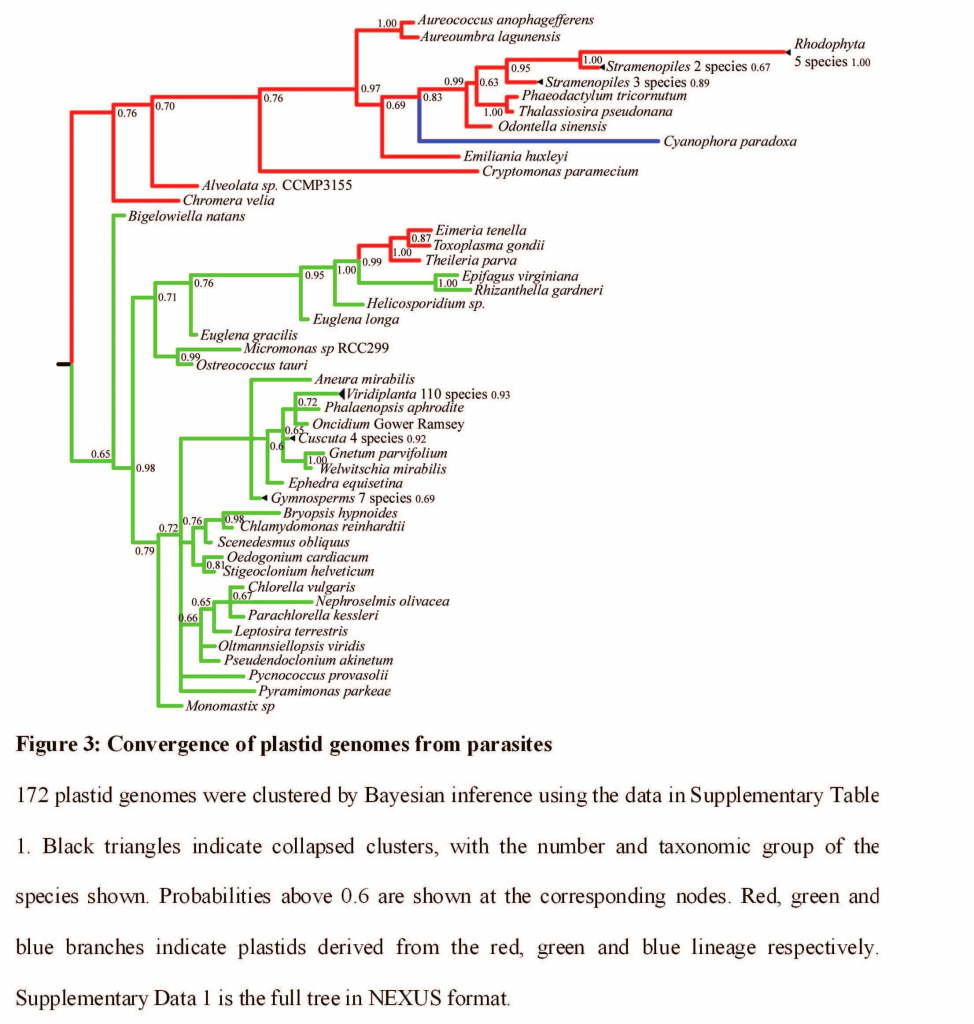
Thanks to convergent evolution, loss of similar genes in parasitic chloroplasts makes it look like Toxoplasma gondii (cause of Toxoplasmosis in humans and cats) is closely related to the plant parasites beechdrops and Western Underground Orchid. Ummm, no.
But wait. Does that mean the chloroplast — the sugar-making machine of plants — does things other than just photosynthesize? Yes. And parasitic plants like R. gardneri are how we know what those things are.
Of the genes that are left on the R. gardneri chloroplast genome, almost all of them encode proteins needed for translation (getting from RNA to protein). Five of the remaining transfer RNAs seem to have remained because either they must interact with many other chloroplast proteins and versions from the main cell would not necessarily recognize the appropriate proteins, or because they have quirky physical modifications that make the chloroplast translation machinery unable to recognize their cellular equivalents. In other words, for these genes, evolution’s backed itself into a corner it’s not easy to get out of with simple chance mutation and natural selection, so instead, it preserves the status quo.
But all these translation-related genes are probably still there for a larger reason: they are necessary to translate the four remaining non-translation related genes on the genome: one making a protein essential for membrane synthesis and two others likely involved in making it, and a fourth gene that makes a protein that seems to have a hand in a variety of important plant processes, at least one of which must be essential. Mutations in the chloroplast’s translation genes are probably fatal because the chloroplast can then no longer make this handful of genes.
Why must these four genes be expressed in the chloroplast and not in the nucleus? The authors hypothesize it has to something to do with a wonderfully vague term called “control by epistasy of synthesis”. As far as I can tell, that means the compartmentalization of these proteins in the chloroplast is essential to their proper synthesis and integration into large protein complexes. So there you have it: the chloroplast may stick around in parasitic plants because it’s handy as a clean manufacturing facility for making parts of a few complicated proteins.
________________________________________________________
* According to the poster of the flickr photograph I used to illustrate this post, Trott donated his specimen to an herbarium for identification and offered £100 to anyone who could find another. Although many looked, none was found until 1979, the year after Trott died. His widow, however, paid up.
![]() Delannoy E, Fujii S, des Francs CC, Brundrett M, & Small I (2011). Rampant Gene Loss in the Underground Orchid Rhizanthella gardneri Highlights Evolutionary Constraints on Plastid Genomes. Molecular biology and evolution PMID: 21289370
Delannoy E, Fujii S, des Francs CC, Brundrett M, & Small I (2011). Rampant Gene Loss in the Underground Orchid Rhizanthella gardneri Highlights Evolutionary Constraints on Plastid Genomes. Molecular biology and evolution PMID: 21289370


{ 1 trackback }
{ 3 comments… read them below or add one }
Just a quick note, there are linear bacterial chromosomes.
Thanks! Good reminder. I’ll fix that now. : )
You can now buy mycorrhizal fungi off the shelf. There are several companies producing this fungi, one of which, Plantworks Ltd, is based in the UK.