Despite appearances, this is not a naughty present from a bachelorette party. It's a peanut worm from France, with its snout tucked inside. Creative Commons Pgrobe
Breaking news: Peanut worms are annelids. Great! I hear you saying. What’s a peanut worm? (And possible sub-questions: What’s an annelid? And why should I care?) Good questions.
Annelids are the bristled, segmented worms. That is, bristles and segments are the synapomorphies for this group, or the shared, derived characteristics that distinguish them from other groups. A synapomorphy makes their grouping both scientifically valid and evolutionarily based*.
To simplify the classic story: Long ago there was a worm that evolved two special new traits its ancestors lacked: bristles and segments. This ancestral worm produced offspring that evolved into all the leeches, hydrothermal tubeworms, polychaete bristle worms, and giant Australian earthworms of the world. As descendants of that first innovative worm, they all have segments. And they all have bristles, although sometimes segments or bristles are hard to see — as in earthworms. They also have parapodia, stumpy little extensions of their body on which the bristles are located. And everyone lived happily ever after, until fish hooks were invented. The End.
Well, almost.
Sometimes, the descendants of a creature that develops a distinctive trait may lose this trait for reasons of evolutionary expedience, or simply because there’s no reason not to. These creatures can fool us into thinking they are *not* a member of the group in question. This often happens with parasites that “de-evolve” because they no longer have to make it on their own; I wrote earlier this year about how microsporidia are actually highly evolved fungi called zygomycetes. We earlier thought they were rather bare-bones parasitic microbes, but their loss of many of the zygomycetes’ unique (derived) characteristics initially fooled us. Well, this has apparently also happened to a quirky little group of marine organisms called peanut worms, or “Sipunculids”.

Possible future constituents of "Sipunculid Worm Jelly". As may be guessed, this is a Chinese delicacy. Creative Commons vmenkov. Click for link.
Peanut worms, like so many other strange but real Earth creatures, are Dr. Seussian in design. My favorite observer of biodiversity after David Attenborough, Colin Tudge, describes them thus:
The phylum Sipuncula includes about 320 species of astonishingly unprepossessing creatures, all marine, some roughly resembling sea cucumbers and others like sprouting potatoes, with their tentacled mouths at the end of the sprouts.
Here’s a picture of a potato-esque peanut worm, and here’s a tentacled snout.
They are called peanut worms because when they retract their long snouts (called introverts) and bunch up, they can look like unshelled peanuts. The peanut worm at the top of this post has its snout mostly retracted, and is a bit peanutty. Some peanut worms also have, and I am not making this up, a little calcareous “anal shield”. I am resisting making a crude joke here. There are several I could choose from and I bet you can guess several. I am being strong. Ahem. Like annelids, they also have trochophore larvae, a key character of the lophotrochozoan mother group (the sister of the skin-shedding ecdysozoa in the protostomes, or invertebrates).
Peanut worms have a number of interesting features which you can see drawn out here: a ringed brain, a long nerve cord (like other annelids), and a helical gut that twists back on itself, positioning the anus somewhat near the mouth. Some live in sediment; some crawl into abandoned shells like sand dollars, where they grow so big they can no longer get out; and some, incredibly, bore into rock. Not bad for a tentacled bag.
Like annelids, peanut worms also have a cuticle-coated epidermis, and two layers of muscle — an outer circular layer and an inner longitudinal layer. They have a true coelom (seel-um), or body cavity. Their skeleton acts on hydrostatic pressure, just like an earthworm. Also like annelids, they have a nuchal organ, or ciliated sensory pit, on their head. The mouth at the end of their snout is surrounded by ciliated tentacles and sometimes hooks. Some also have ocelli, or very simple eyes.
We have a few rare fossils of actual sipunculids from the Cambrian(see awesome photos buried in article) — 520 million years ago — and they look remarkably similar to modern peanut worms. Sharks(which have remained similar in for for a mere 420 million years): eat your hearts out. This just goes to show the pace of evolution varies in its own good time. Peanut worms surely evolved during all this time too. They just did so many times slower than uppity groups like vertebrates, because apparently there was little selective pressure to do so.
Amusingly, one of the earliest described species of peanut worm was named Golfingia macintoshii by E. Ray Lankester since he dissected the first specimen provided by a certain Professor Mackintosh between rounds of golf at Saint Andrew’s in Scotland (which for some reason, as an atom in the universe of golf trivia, reminds me of the legendary invention of golf by the Bullroarer Took at the Battle of Greenfields). Golfingia is still the name of a major genus of the organisms.
OK. So now I’ve convinced you (I hope) these things are cool but odd. They share a lot in common with annelids. But also a lot not — no segments. No bristles. No parapodia.
Hmm. Taxonomic impasse. Genes to the rescue!
In a study in Nature released last week, scientists compared the DNA of a variety of peanut worms, spoon worms, and annelids. In this study, scientists used only sequences from expressed genes only. They did this by taking the messenger RNA, an edited form of DNA used to make proteins, and making DNA directly from it (called cDNA, or complementary DNA). They then sequenced this, as DNA pre-edited to make functional proteins is more likley to have meaningful changes to its code due to selective pressure. Bits of non-translated DNA that are edited out are free to mutate much more rapidly and randomly, dampening the signal of evolution.
Scientists can use this method because over time, more closely related organisms will have more similar genetic information. Comparing the sequences of one gene in three creatures to make a simple relatedness tree would be easy for a human, but this study looked at the amino acids (the building blocks of proteins) coded for by the DNA at 47,953 positions in 34 annelid groups. Computers more easily crank through these sorts of hyper-space logic problems, and they did here too. Based on this analysis, the peanut worms fit cozily inside the annelids. When we had only their appearance to go by, we were confused. But with DNA, in this case at least, the situation becomes much clearer.
Sooooooo — to sum up: up until now, scientists suspected peanut worms were in the same branch of bilaterally symmetrical animals as annelids, the lophotrochozoa (who either have crowns of cilia called lophophores or trochophore larvae), but not actually annelids themselves. You can see where the peanut worms (sipunculids) used to fit in the lophotrochozoans here. Now, scientists see that they’re not just lophotrochozoans, they’re also our old friends the annelids.
Upending the Annelid Family Tree
But the biggest news from their results was not that peanut worms are annelids (although that is good to know), but that our traditional taxonomy of annelids is wrong — and that an old, dusty classification for annelids was right after all. The current system divided all the annelids into the clitellata, or worms that have a collar called the clitellum (that ring you see on earthworms), and the polychaeta, or bristle worms (which, as I’ve written before, have big, showy bristles). But it turns out that clitellata as a group is embedded within the polychaete worms, and that the polychaete worms have non-polychaete worms like peanut worms (which lack bristles, parapodia, or segements, because they lost them) and another non-polychaete that is a weirdo parasite of sea lillies — the myzostomida, which I wrote about here — embedded within them. That makes polychaeta polyphyletic, which is a dirty, dirty word to taxonomists. It is a group that includes an ancestor and some, but not all of its descendents. Hence, polychaeta’s gotta go.
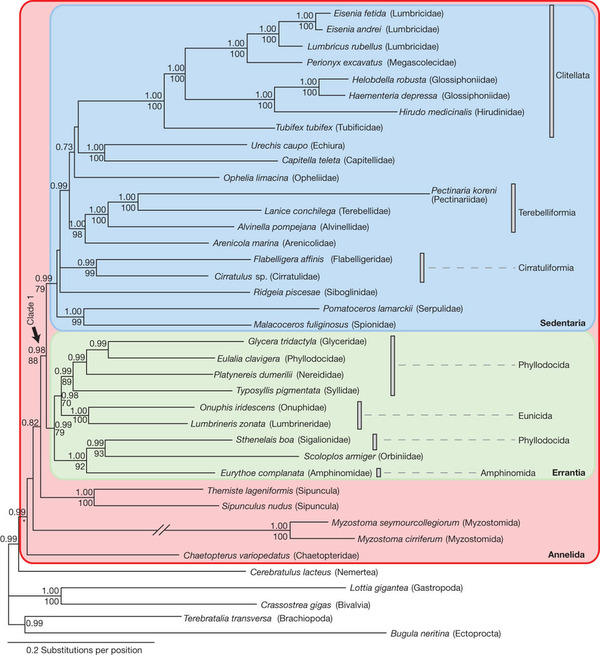
Don’t be frightened by this diagram — it just shows a geneaology of annelids, just like your family tree. The numbers on the tree indicate how certain the scientists/computers feel about their branching hypotheses; numbers close to 1.00 or 100 mean they are very highly confident that that hypothesis of relatedness is correct. And as you can see, with so much data, the scientists are very confident about this tree.
The *new-old* classification supported by the genes was proposed way back in 1865 (though it excluded the earthworms and leeches) by Jean Louis Armand de Quatrefages de Bréau. It is based not on bristling, but on life history — sedentary worms versus active worms. But later scientists dismissed it as the product of another dirty word: convergent evolution — the force that make whales and sharks both seem like they might be a lot more closely related than they are. Quoth the article “This systematization was dismissed in the 1970s as being arbitrary groupings useful only for practical purposes.” Ouch. Well, it turns out that was wrong.
The clitellata(collared/ringed annelids) are still supported as a true monophyletic (ancestor and all its descendants — distinguished by their synampomporphies; taxonomists like these) group. But there are two new groupings: the Sedentaria, which means what it sounds like: things that burrow, or live in tubes, sucking mud or lazily filtering water with tentacles and who could generally could lay around watching re-runs of “The West Wing” for the rest of their lives and not have to worry about their BMI. Things like earthworms, and deep-sea hydrothermal tube worms.
The Errantia are similarly named — they are errant creatures that swim, writhe, and actively pursure their prey or nosh on big algae. Things like the tentaculate and green-bomb-throwing ex-polychaetes I’ve written about here before. On the fringe of these two groups we have some organisms that are neither — like peanut worms — but are still annelids based on their genes and morphology. And there you have it.
Once you have a tree like this, it’s a fairly simple logic problem to determine what the ancestral form that existed way back on each of those branch points must have looked like. You just look at its descendants and see what they have in common. And here is the drawing the scientists came up with for the ancestral annelid (last common ancestor, scientsts would say) and the ancestral Clitela and Errantia.
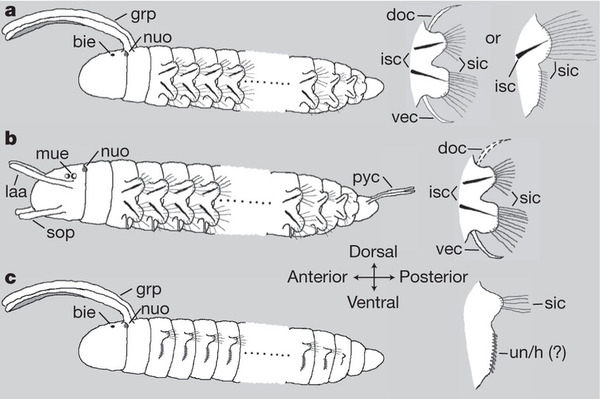
(a) shows features the first annelid likely possessed, (b) the first Errantian, and (c) the first Sedentarian. Note they all have parapodia, though the bristles are much shorter and feebler in Sedentarians, and the parapodia are not supported by stiff internal bristles (chaetae: kee-tee).
 As you can see, they have determined that that first annelid worm (a) was indeed segmented, since peanut and another unsegmented annelid I did not cover — the spoon worms, or Echiura — are embedded within segmented annelids, not both at the base of the tree.
As you can see, they have determined that that first annelid worm (a) was indeed segmented, since peanut and another unsegmented annelid I did not cover — the spoon worms, or Echiura — are embedded within segmented annelids, not both at the base of the tree.
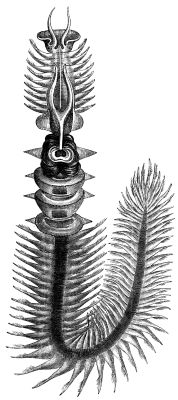
The annelid in the earliest branching position — Chaetopterus, or the parchment worm (see left) — is not only segmented, but quite complicatedly so — it has three different sections of segments on its body.
So it seems the peanut worm simply found that, although segments and bristles were awesome, they were costly options it simply didn’t need to get the job done in its little marine habitats, where it has happily existed more or less as-is, and perturbed by little except predators and Chinese gourmands, for over 500 million years.
———————————————————————–
* Modern taxonomy attempts to base groupings on evolutionary history, since it only happened one way and once. Any other classification system would be arbitrary
 Struck, T., Paul, C., Hill, N., Hartmann, S., Hösel, C., Kube, M., Lieb, B., Meyer, A., Tiedemann, R., Purschke, G., & Bleidorn, C. (2011). Phylogenomic analyses unravel annelid evolution Nature, 471 (7336), 95-98 DOI: 10.1038/nature09864
Struck, T., Paul, C., Hill, N., Hartmann, S., Hösel, C., Kube, M., Lieb, B., Meyer, A., Tiedemann, R., Purschke, G., & Bleidorn, C. (2011). Phylogenomic analyses unravel annelid evolution Nature, 471 (7336), 95-98 DOI: 10.1038/nature09864
Arendt, D. (2011). Evolutionary biology: Annelid who’s who Nature, 471 (7336), 44-45 DOI: 10.1038/471044a