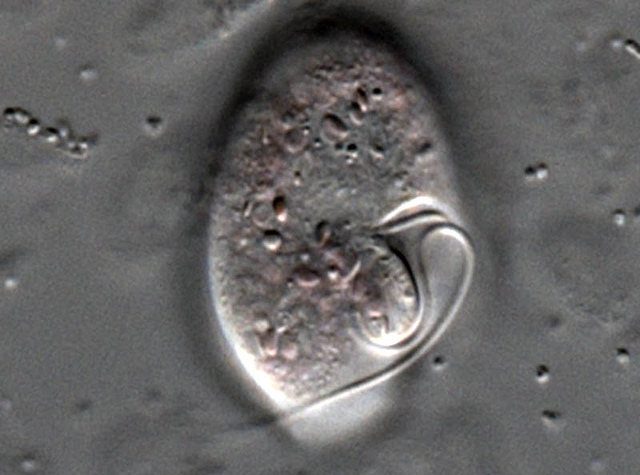
I would like a fascinator made in this shape. The dinoflagellate Oxyrrhis marina with its transverse and longitudinal flagella. Creative Commons Census of Marine Life E&O
Life on Earth is full of weird convergences. For example, the cell walls of fungi are made of chitin, as are the shells of insects and arthropods like lobsters and crabs. Why? Whether due to chance or something deeper, I have never heard a good explanation.
Similarly, the proteins called rhodopsins are found in two seemingly completely unrelated places: the retina of your (and all vertebrate) eyes, and in some photosynthetic archaea and bacteria. We use ours for detecting light (it’s in the rod cells used for low-light vision); they use theirs to pump protons (as illustrated and discussed recently here) to make food.
And now, scientists have discovered a predatory dinoflagellate that has apparently stolen a bacterial photorhodopsin from one of its meals — and is using it (see also here).
The protein in question is called rhodopsin (Greek root “rhodo” = rose and “opsis” = sight) because it absorbs blue-green light and so appears purplish-red. Rhodopsins have seven protein coils called alpha helices that pass through membranes the protein is embedded in. In turn, embedded inside the helices is retinal, a light-sensitive pigment. As a result, the whole structure changes shape in response to light. In vertebrates, rhodopsin has another subunit called a “G protein“. G protein acts as signal transducer, or on-off switch in a signaling cascade — just like a switch in an electrical circuit. Vertebrates use it to relay information to the brain about what the eyes are seeing. Bacteria use their rhodopsin simply for pumping protons, not for signalling, so they have no need for G protein section.
The bacterial protein — still seven alpha helices and a retinal — resembles overall shape of vertebrate protein, so scientists suspect they may be distantly related. Proteorhodopsin — the bacterial version, was only discovered in 2000, but the (now ironically named) bacteriorhodpsin found in archaea was discovered much earlier, in archaea living and photosynthesising in sun-drenched salt flats, brackish pools, or salt marshes.
The dinoflagellate Oxyrrhis marina (ox-EE-ris MARE-i-na, I think) is neither a vertebrate nor a prokaryote (bacterium/archaeon). And yet O. marina makes so much rhodopsin it has turned pink. How? Scientists believe the gene for the proteorhodopsin was acquired by what biologists call horizontal gene transfer — that is, O. marina was happily digesting its photosynthetic bacterial prey when the gene for this protein somehow wandered off and found its way into the nucleus (where DNA is stored) and slipped, spliced, or got sliced into the chromosome.
This appears to be a case of “hedging your bets” because O. marina is now both photosynthetic and predatory. So in this case, you can make your own cake and stalk it too. Scientists believe they use this protein not only to make energy by pumping protons and passing them back down a chemical gradient via an ATP synthase, but also to help digest the very prey they stole it from. As they say, all too easy.
But before I say more, a few words on what dinoflagellates are are in order.
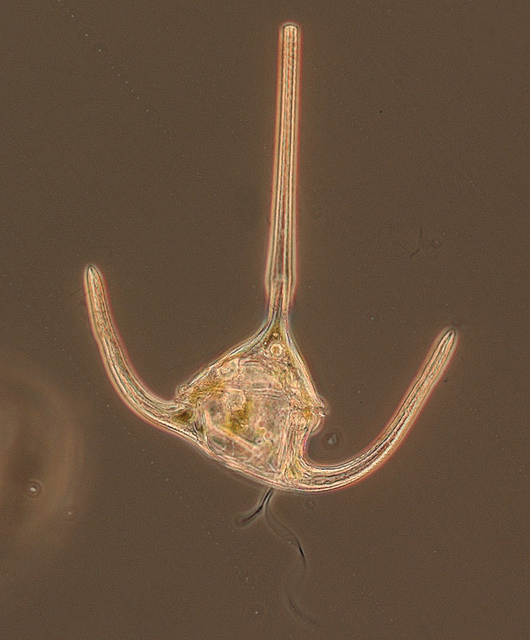
The dinoflagellate Ceratium longipes, in all it's tricorne glory. One, and possibly two flagella visible. Creative Commons Census of Marine Life E&O
In short, dinoflagellates are two-tailed plankton. They are also protists, the loose association of single-celled organisms with DNA inside nuclei and cellular organelles that are usually much bigger than bacteria or archaea. About half are predatory, half make their own food, and obviously, now we know some do both. The photosynthetic lot are the second most abundant constituent of the photosynthetic marine plankton after diatoms (which I covered here).
Dinoflagellates (from the roots for “whirling whip”) are also alveolates like ciliates (including the paramecia I wrote about here) and apicomplexans (which include Plasmodium, the protist that causes malaria). That means they often have sacs called alveoli under their cell membranes, trichocysts (defensive spikes that shoot out like harpoons), and tubular mitochondrial folds, or cristae. Some even produce structures like the nematocysts of jellyfish. You can explore how dinoflagellates fit into the life family tree here (use the black arrows to move toward the root).
Some dinoflagellates cause the poisonous red tides infamous for sickening fish and swimmers alike. Others build up in tropical fish or shellfish and cause ciguatoxin or paralytic shellfish poisoning in people. Others become the zooxanthellae, the green algal symbionts found in coral and other animals and protists. These photosynthetic dinoflagellates vastly increase the speed at which corals can build their skeletons. And finally, some dinoflagellates (including some red tiders) are bioluminescent, emitting short flashes of light when disturbed, and are responsible for the unearthly glow of the wakes of ships passing through tropical waters in the night.

Bioluminescent red tide dinoflagellates getting all bright and bothered by rolling surf. Creative Commons Catalano82. Click for link.
Dinoflagellates have some interesting quirks; their chromosomes stay condensed throughout the cell cycle and down relax into a spaghetti pile of chromatin (individual DNA threads) between cell divisions. And they don’t wrap their DNA around proteins called histones as most of the rest of us upstanding eukaryotes do. They prefer instead to attach it to the inside of the nuclear membrane. Also, they tend to eschew the reliable DNA base thymine in favor of the boutique 5-hydroxymethyluracil.
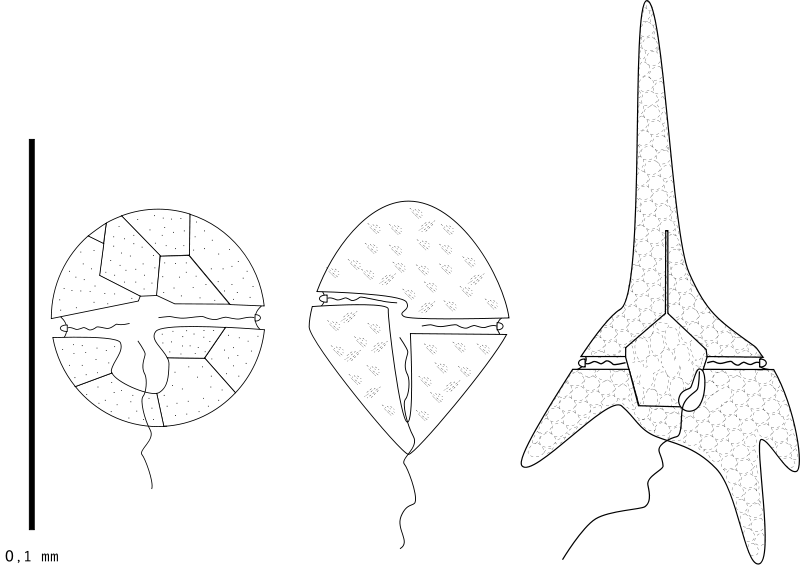
My plans to take over my neighborhood swimming pool are now complete . . . Dinoflagellate blueprints. Creative Coimmons Shazz. Click image for link.
Their two flagella — which emerge from the same point — are often set in grooves. One is a belt-like transverse groove called the cingulum, and the other is a longitudinal groove called the sulcus. You can see those grooves in a beautiful scanning electron micrograph here. (See also here and here for a dinoflagellate that I swear resembles Baron Harkonnen in his fat suspensor suit). The flagellum set in the cingulum wraps all the way around, while that set in the sulcus trails off the back like a rudder. Strangely, the transverse flagellum is responsible for most of the forward motion and is what sets dinoflagellates whirling, while the longitudinal chiefly helps steer.
Dinoflagellates are also really good at engulfing photosynthetic organisms and endosymbi-izing them. Most of their chloroplasts have three membranes, suggesting they came from an ingested alga, not a bacterium, and are the result of a secondary engulfment, or endosymbiosis. Others have chloroplasts of different color, shape and form, some still with nuclei. Thus we can infer that dinoflagellates are really good at finding themselves new photosynthetic dates by hook or crook — definitely crook, in the case of Oxyrrhis.
Flexibility and Finding a way seem to be themes with dinoflagellates. O. marina, for example, is common in shallow tide pools around world; it will go so far as to cannibalize its own species if it comes to it and can take down prey almost as big as it is. Photosynthesis. . . hunting . . . cannibalism — this thing is the MacGyver of protists. It’s going to survive with whatever comes to hand. So if you’re ever doing O. marina research, please do us all a favor: do not keep bubble gum, paper clips, or high explosives anywhere near your experimental organisms. Thank you.

{ 1 trackback }
{ 1 comment… read it below or add one }
Now I know what a fascinator is. So informative. :)